A rare and “highly drug resistant” bacteria in recalled eyedrops is continuing to cause problems across the country.
At least three have died and nearly 70 patients have been impacted in 16 states. There have been reports of vision loss, and some people even losing an eye.
The bacteria is a rare strain of Pseudomonas aeruginosa found in eyedrops by Exricare and Delsam Pharma that were recalled in February, according to the CDC.
EzriCare Artificial Tears was the brand most commonly reported, and the FDA recall can be found below:
Company Announcement
Global Pharma Healthcare is voluntarily recalling all lots within expiry of their Artificial Tears Lubricant Eye Drops, distributed by /EzriCare, LLC- and Delsam Pharma, to the consumer level, due to possible contamination. The Centers for Disease Control and Prevention (CDC) alerted FDA to an investigation of a multi-state cluster of Verona Integron-mediated Metallo-β-lactamase (VIM)- and Guiana-Extended Spectrum-β-Lactamase (GES)- producing carbapenem-resistant Pseudomonas aeruginosa (VIM-GES-CRPA) infections possibly associated with the use of the artificial tears manufactured by Global Pharma Healthcare. To date, there are 55 reports of adverse events including eye infections, permanent loss of vision, and a death with a bloodstream infection.
Risk Statement: Use of contaminated artificial tears can result in the risk of eye infections that could result in blindness.
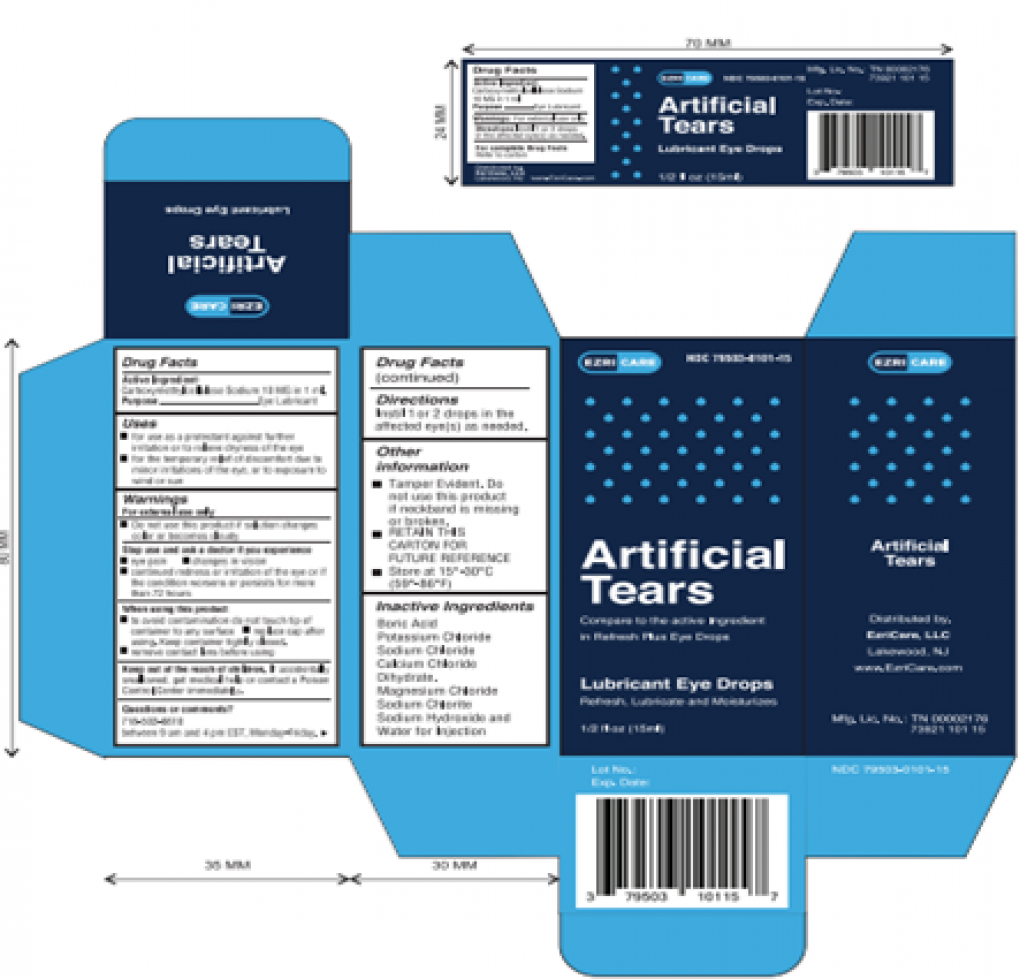
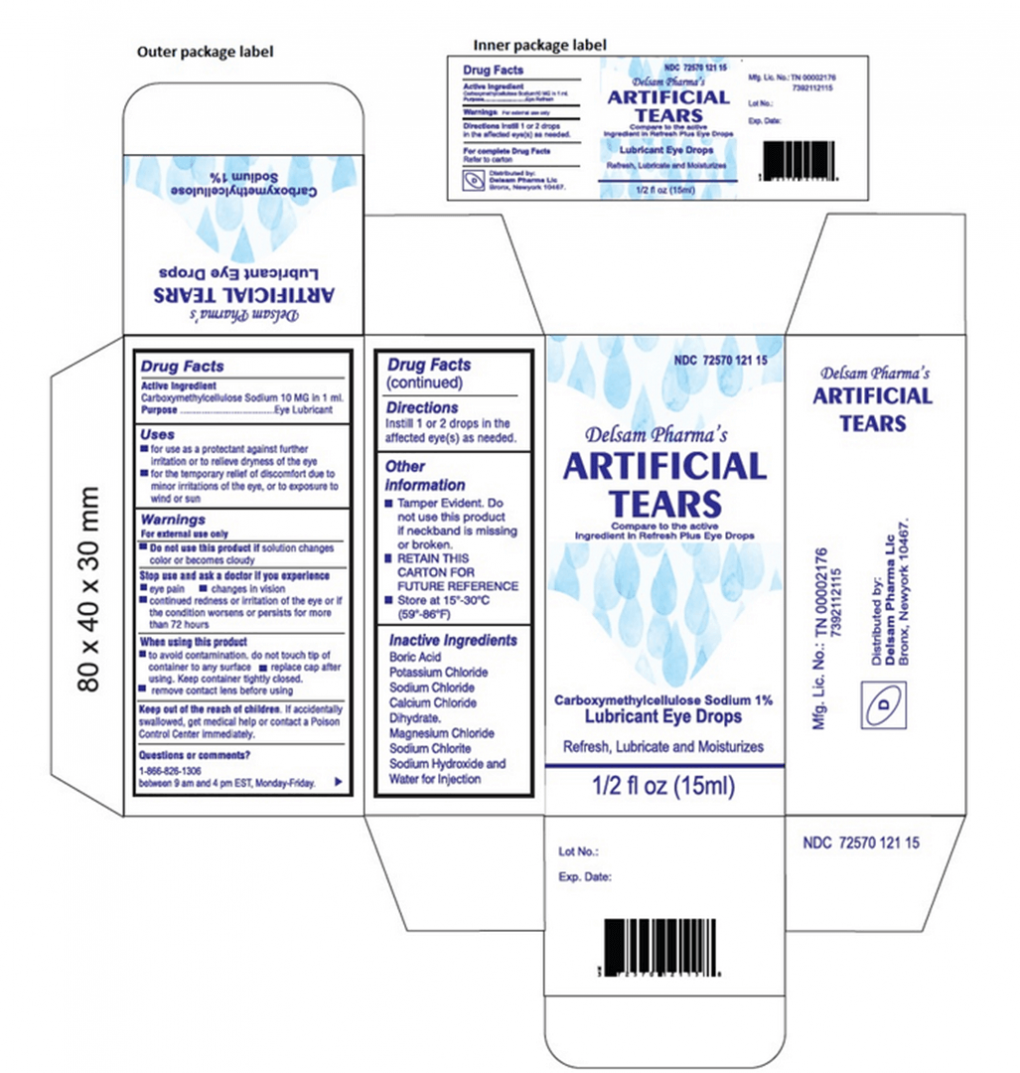
Artificial Tears (carboxymethylcellulose sodium) Lubricant Eye Drops, 10 mg in 1 mL, ½ fl oz (15 ml) bottle are used as a protectant against further irritation or to relieve dryness of the eye for the temporary relief of discomfort due to minor irritations of the eye, or to exposure to wind or sun. The product is packaged in a bottle with a safety seal and are placed in a carton box Ezricare NDC 79503-0101-15, UPC 3 79503 10115 7; Delsam Pharma’s NDC 72570-121-15, UPC 3 72570 12115 8. It can be identified by the photos below. The product was distributed Nationwide in the USA over the Internet.
Global Pharma Healthcare is notifying the distributors of this product, Aru Pharma Inc. and Delsam Pharma and is requesting that wholesalers, retailers and customers who have the recalled product should stop use.
Consumers with questions regarding this recall can contact the distributors: Aru Pharma/Ezricare, LLC by phone: 1-516-715-5181 or by e-mail: arupharmainc@yahoo.com from Monday to Friday, 11am to 4pm EST; or DELSAM Pharma LLC by phone: 1-866-826-1306 or by e-mail: delsampharma@yahoo.com from Monday to Friday from 11am to 4pm EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these over-the-counter drug products.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- DELSAM Pharma LLC, Aru Pharma/Ezricare, LLC
- 1-866-826-1306, 1-516-715-518
- delsampharma@yahoo.com, arupharmainc@yahoo.com
Product Photos