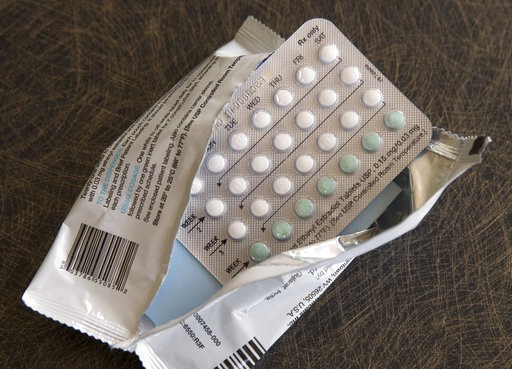
The FDA says there is a voluntary nationwide recall of a birth control pill.
The Apotex Corporation issued the recall, saying four lots of specific pills might possibly contain defective blister packages with incorrect arrangements and/or an empty blister pocket. As a result, users might miss a dose, or take a placebo when they think they are taking an active tablet. No cases of unintended pregnancy have been reported.
The affected pills: Drospirenone and Ethinyl Estradiol Tablets, USP may possibly contain defective blisters with incorrect tablet arrangements and/or an empty blister pocket. As a result, users may not take a tablet due to a missing tablet or take a placebo instead of an active tablet, causing an adverse event or even pregnancy. No case of unintended pregnancy has yet been reported, according to Apotex.
Users with questions regarding this recall are urged to contact their pharmacy and healthcare provider for medical advice and return the impacted packages to their pharmacist.
Drospirenone and Ethinyl Estradiol is an estrogen/progestin combination oral contraceptive indicated for the prevention of pregnancy. The tablets in the inner carton are arranged as 21 active yellow tablets followed by seven white placebo tablets.
The following lots have been recalled: 7DY008A, 7DY009A, 7DY010A, and 7DY011A. The outer carton displays NDC# 60505-4183-3 and contains three inner cartons (NDC# 60505-4183-1). The expiration date is listed as 8/2020.
For more information call 800-706-5575 or visit Apotex.com. Users can also read more about this recall by clicking the link here.
The affected product is manufactured by Oman Pharmaceutical Products Co. llc. Oman under the subcontract from Helm AG, Nordkanalstrasse 28, Hamburg, 20097, Germany.